ISO 13485 Consulting & Certification Services in Australia – Medical Devices Quality Management
TopCertifier is a leading ISO certification consultancy that has assisted numerous organizations across Australia in achieving ISO 13485 certification. Our team of highly experienced consultants specializes in Quality Management Systems for medical devices and ISO standards. With deep expertise in ISO 13485, we provide expert guidance and comprehensive support throughout the certification process.
We work closely with companies in Sydney, Melbourne, Brisbane, Perth, Adelaide, and other cities to ensure that their Medical Device Quality Management System (MDQMS) is effectively implemented and maintained in full compliance with ISO 13485 requirements. Our services include gap analysis, documentation support, internal auditor training, and audit preparedness to equip your team for certification. Every engagement is tailored to your operational and regulatory needs.
Choosing TopCertifier as your ISO 13485 certification consultant in Australia ensures a smooth and reliable certification process. Our consultants bring in-depth knowledge of both the ISO 13485 standard and the medical device industry. We help you develop efficient, compliant QMS processes that meet national and global regulatory expectations. Our customized solutions provide medical device manufacturers, suppliers, and service providers in Australia with a streamlined path to ISO 13485 certification.
ISO 13485 Certification Services in Australia Offered by TopCertifier
ISO 13485 Gap Analysis:
Conduct a detailed gap analysis to identify areas where the organization’s existing Quality Management System does not meet ISO 13485 requirements for medical devices.
ISO 13485 Documentation Review:
Review and assess current documentation, including quality manuals, procedures, and work instructions, to ensure alignment with ISO 13485 and applicable Australian regulatory guidelines.
ISO 13485 Process Improvement:
Help Australian medical device organizations enhance their quality processes by identifying inefficiencies and integrating continuous improvement strategies for full ISO 13485 compliance.
ISO 13485 Training:
Deliver tailored training sessions on ISO 13485 requirements, covering quality principles, risk management, documentation, and compliance practices relevant to the Australian medical device sector.
ISO 13485 Internal Audits:
Conduct internal audits to assess whether the QMS is being effectively implemented and maintained in accordance with ISO 13485 and Australia’s medical device regulations.
ISO 13485 Certification Audit Support:
Support your organization throughout the external certification audit with mock audits, documentation preparation, and guidance to resolve any non-conformities during auditor interaction.
ISO 13485 Lead Auditor Training:
Provide professionals in Australia with the knowledge and skills to plan, execute, and manage ISO 13485 QMS audits in accordance with ISO 19011 and industry-specific requirements.
ISO 13485 Lead Implementer Training:
Equip Australian professionals with the tools to design, implement, and maintain an ISO 13485-compliant QMS tailored to the regulatory and operational needs of the medical device industry.
Trust Us To Lead The Way In Certification And Compliance
Knowledge And Expertise
Thorough Understanding Of The Framework, Its Requirements, And Best Practices For Implementation
Proven Track Record
Successful Track Record Of Helping Clients Achieve Compliance, With Positive Client Testimonials And Case Studies.
Strong Project Management Skills
Ensure The Compliance Engagement Runs Smoothly And Is Completed On Time And Within Budget.
Experienced Team
Possession Of Experienced Professionals, Including Auditors, Consultants, And Technical Experts
Exceptional Customer Service
Committed To Excellent Customer Service With Clear Communication, Responsive Support, And A Focus On Satisfaction.
Competitive Pricing
We Prioritize Delivering High-Quality Services With Competitive Pricing That Provides Exceptional Value To Our Clients
FAQs
FREQUENTLY ASKED
ISO 13485 is an international standard that defines the requirements for a Quality Management System (QMS) specific to the medical devices industry. It ensures that companies involved in the design, production, installation, and servicing of medical devices consistently meet both customer and regulatory expectations in Australia and abroad.
ISO 13485 compliance is vital for Australian medical device companies as it demonstrates their ability to manufacture safe and effective products. It enhances quality, aligns with regulatory frameworks, improves risk management, and is a key requirement for accessing both domestic and global medical device markets. It also builds trust with regulators, partners, and end-users.
Regulatory Acceptance: Supports compliance with Australia’s TGA (Therapeutic Goods Administration) and international regulations.
Improved Product Quality: Promotes consistency, traceability, and safety in manufacturing.
Market Access: Enables entry into Australian and global medical device markets.
Operational Efficiency: Reduces errors, streamlines procedures, and optimizes performance.
Customer Confidence: Builds trust among healthcare providers, patients, and stakeholders.
Competitive Advantage: Distinguishes your organization in the highly regulated medical industry.
ISO 13485 is applicable to:
- Medical device manufacturers in Australia
- Component suppliers and service providers
- Distributors and importers
- Sterilization and packaging services
- Design and development firms
- Testing and calibration laboratories
The cost of ISO 13485 certification in Australia depends on:
- Organization size and structure
- Number of sites and product types
- Current level of compliance
- Need for consulting, training, and documentation
- Choice of certification body
Typically, ISO 13485 certification in Australia takes 3 to 6 months depending on:
- The organization’s existing QMS maturity
- Availability of internal resources
- Readiness of documentation
- Training needs and complexity of operations
- Timelines of the selected certification body
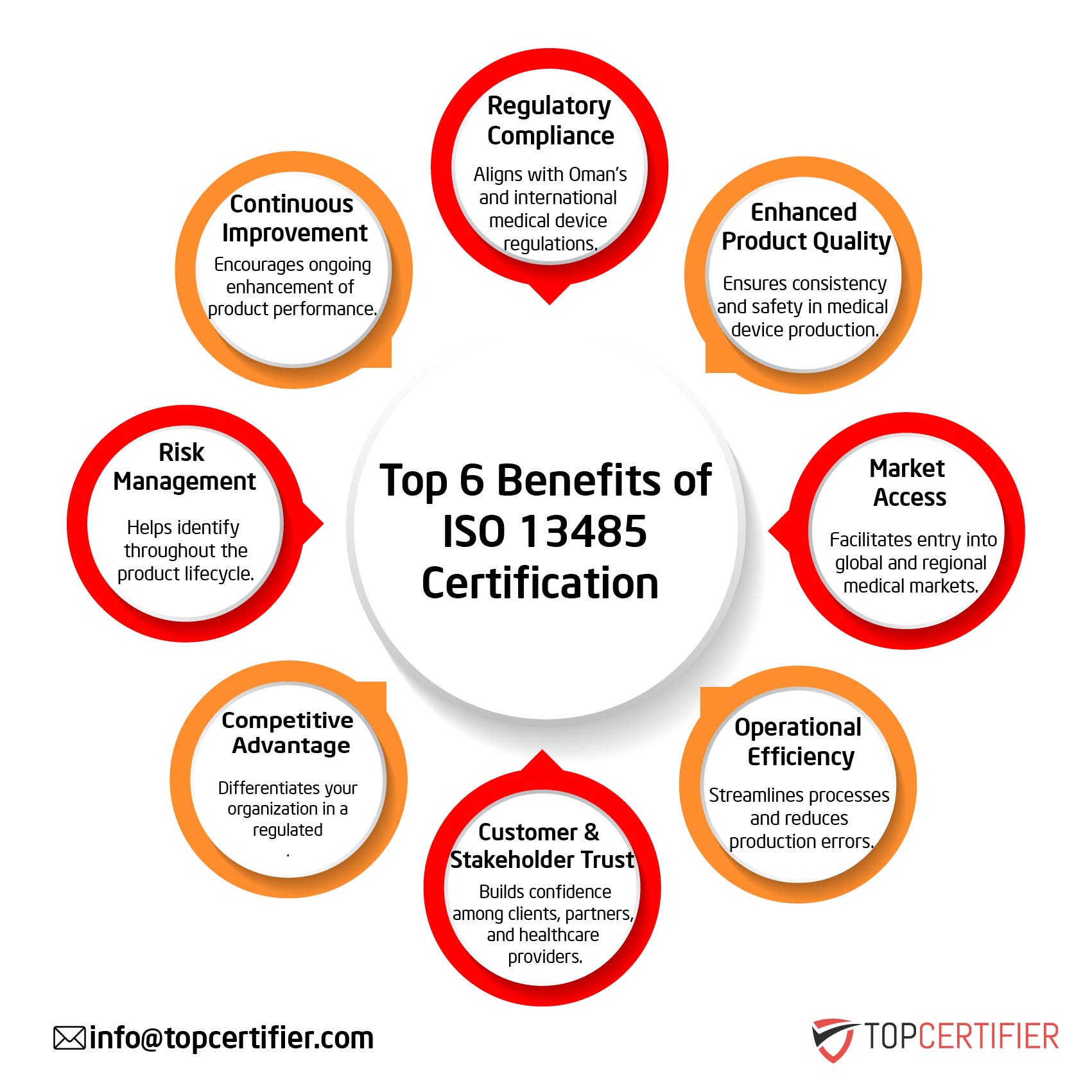
- Regulatory Compliance: Aligns with TGA and international requirements.
- Enhanced Product Quality: Ensures safety and reliability of devices.
- Market Access: Opens entry to global and regional medical markets.
- Operational Efficiency: Reduces errors and optimizes production workflows.
- Customer & Stakeholder Trust: Builds confidence among users and partners.
- Competitive Advantage: Strengthens brand value in a regulated industry.